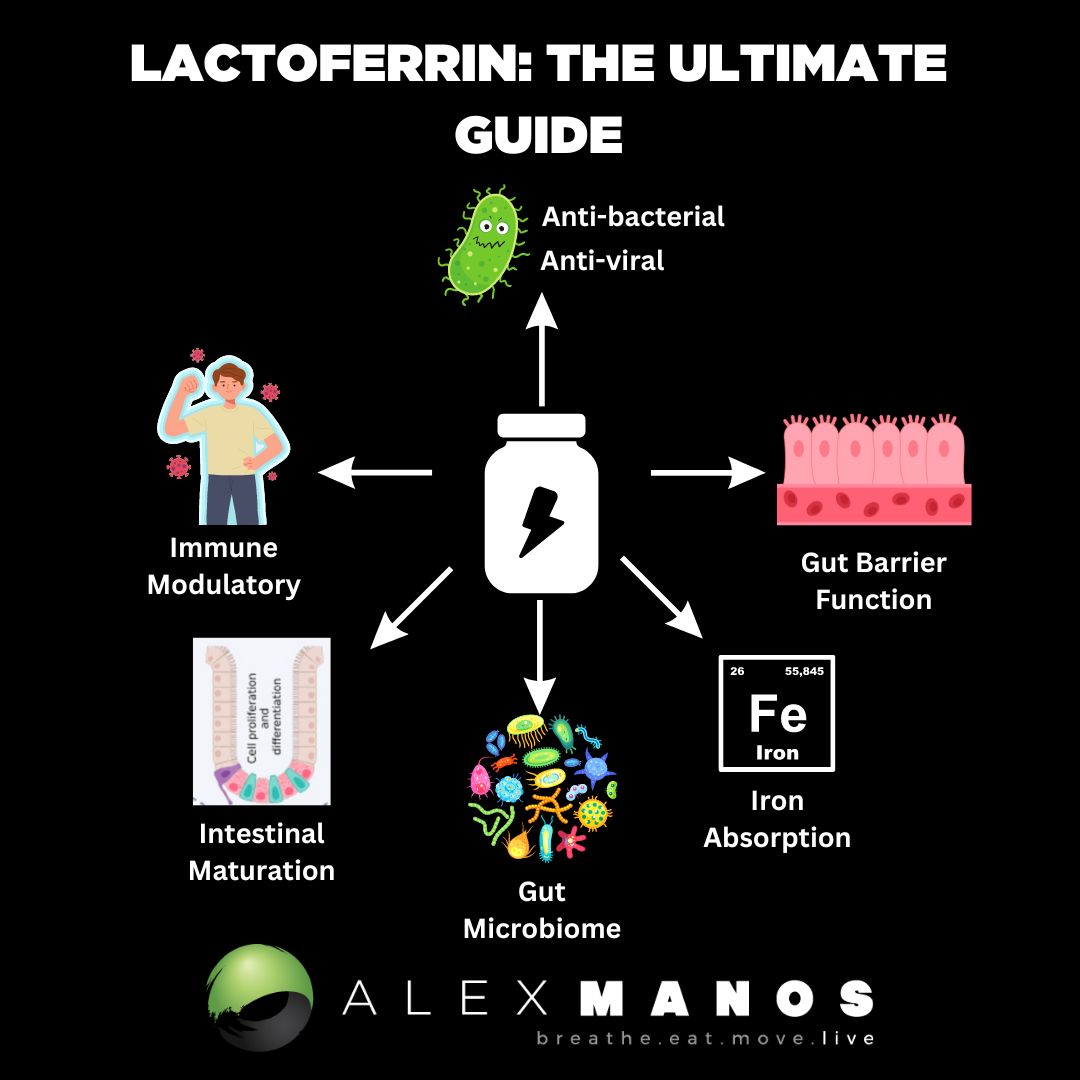
What Is Lactoferrin?
Lactoferrin is a glycoprotein found in breast milk.
What’s a glycoprotein you ask. It’s a molecule that comprises protein and carbohydrate chains.
Lactoferrin has been found to modulate the immune system and thus inflammation, have antioxidant, anti-tumour, and antimicrobial activities; enhance iron absorption; and elicitation of cellular responses, including activation, differentiation, and proliferation. (source)
Lactoferrin And Leaky Gut
Lactoferrin also has protective effects on intestinal barrier function; to maintain the dynamic balance between the body and the intestinal tract, a complete barrier system is crucial. Lactoferrin has been reported to enhance the barrier function of a Caco-2 cell layer damaged by bacterial lipopolysaccharide (LPS). (source)
Lactoferrin and Intestinal Growth and Maturation
The significant growth and development of the gut lining during the first years of life is the result of an equilibrium between proliferation and differentiation. The balance between proliferation and programmed cell death (apoptosis) produces a controlled increase in cell population. This balance is especially important in the small intestine, where continuous processes of cell elimination and replacement occur.
Lactoferrin has been suggested to be a gut development modulator, through direct effects on intestinal epithelial cell proliferation and differentiation during infancy.
Lactoferrin Repairs Mycotoxin Induced Damage
Lactoferrin was also suggested as an alternative treatment for leaky gut as the result of aflatoxin M1 (a mycotoxin) damage. The protective effects of Lactoferrin on the intestinal barrier dysfunction were related to two types of signalling pathways:
- One related to epithelial cell viability.
- One with intestinal integrity.
All the pathways associated with cell viability have been reported to be involved in the process by which Lactoferrin stabilises the intestinal barrier function. In the pathways associated with the intestinal integrity the endocytosis, tight junction, adherens junction, gap junction, focal adhesion and ECM-receptor interaction are participating. (source)
Regulation of the Innate Immune System
Lactoferrin exerts its effects on the innate immune system through cellular and molecular mechanisms. At the cellular level, Lactoferrin can interact with different immune cells, as LFRs have been identified in lymphocytes, macrophages, and dendritic cells, triggering cellular responses such as migration, maturation, and proliferation. It has been shown that Lactoferrin promotes dendritic cell maturation, enhances natural killer (NK) cell activity, increases neutrophil migration, and induces macrophage activation, increasing their phagocytic capacity. Iigo et al. observed that patients with colorectal polyps, who ingested bLF, had an increased number of NK cells in their polyps. (source)
Accumulating studies, with animal and human models, report that lactoferrin can be considered a potent anti-inflammatory for the prevention and treatment of gastrointestinal inflammatory diseases. This protein helps to limit excessive inflammatory responses by inhibiting the production of pro-inflammatory cytokines, such us TNF-α, IL-1β, IL-6, and IL-8, and by stimulating the synthesis of anti-inflammatory cytokines including IL-4 and IL-10. (source)
The reason for this effect of Lactoferrin seems to be associated with different mechanisms of action. On the one hand, LF can modulate the inflammatory response by binding to lipopolysaccharide (LPS) on Gram-negative bacteria, or to the soluble (sCD14) or membrane (mCD14) receptor. This interferes with the formation of the LPS–CD14 complex, resulting in an attenuation of the Toll-like receptor 4 (TLR-4) signalling pathway. The interaction of LPS with TLR-4 induces intracellular biochemical signalling that concludes with the release of pro-inflammatory cytokines, which, when produced in excess, can lead to tissue damage. (source)
Therefore, through their LPS-binding properties, lactoferrin can counteract the LPS-induced inflammatory response, as well as the intestinal damage. On the other hand, lactoferrin may act as a novel transcriptional regulator through Lactoferrin receptors located on the brush border membrane of the small intestine. After lactoferrin binds to its receptor, it is internalised into intestinal cells by endocytosis and subsequently translocated into the nucleus. Inside the nucleus, lactoferrin binds to specific locations on DNA, thereby acting as a transcription factor, regulating the expression of various genes, including those involved in the inflammatory response. (source)
Lactoferrin may also act as a potent anti-inflammatory agent by scavenging reactive oxygen species (ROS), which are strongly produced by granulocytes in the course of inflammation. ROS induce inflammation through their ability to increase the production of IL-8 in intestinal epithelial cells. Therefore, an excess of these species enhances the inflammatory response, which contributes to the appearance of clinical symptoms. Lactoferrin is able to maintain the physiological balance of ROS levels by chelating free iron, which is essential for their production, or by regulating key antioxidant enzymes. (source)
Lactoferrin as Fecal Biomarker of Inflammation
During intestinal inflammation, polymorphonuclear neutrophils infiltrate the mucosa and release proteins and cytokines. Among the proteins secreted by neutrophils, we can find lactoferrin, which is the main component of neutrophil secondary granules. This protein is released by degranulation during an inflammatory process, resulting in increased levels of lactoferrin in fecaes, and its concentration is proportional to neutrophil translocation to the digestive tract. Consequently, as with calprotectin, this protein can be used as a faecal biomarker of intestinal inflammation.
LF and calprotectin have proven their value in detecting active IBD, predicting disease recurrence, or assessing responses to medical treatment. Although calprotectin is ordered much more frequently, studies evaluating lactoferrin in the diagnosis of IBD show that calprotectin and lactoferrin results have high concordance. In fact, specific antibodies against both proteins are used together in some commercial tests to detect IBD. (source)
Elevated faecal LF levels have been found in patients with ulcerative colitis and Crohn’s disease, with sensitivities ranging from 78–90% and specificities from 90–100%. In addition, lactoferrin concentrations show significant correlation with endoscopic assessment of intestinal injury; therefore, patients with more severe endoscopic disease activity have higher lactoferrin concentrations in faeces. This biomarker is also useful to screen for inflammation associated with IBD versus irritable bowel syndrome (IBS).
Regarding infectious diarrhoea, some authors have suggested the use of faecal lactoferrin as an important tool to predict and monitor the clinical severity and course of gastrointestinal infections. In Cryptosporidium parvum-infected children, growth deficits were associated with the degree of oocyst excretion and lactoferrin positivity. Moreover, increased levels of faecal lactoferrin were linked to a greater number of days and episodes of diarrhoea in patients with Giardia duodenalis infection. (source)
Chen et al. found that concentrations of faecal lactoferrin increased during bacterial infection (Salmonella and Campylobacter) and with higher severity of illness, compared to patients with viral infections and mild disease activity.
Modulatory Effects of Lactoferrin on Gut Microbiome
The intestinal microbiome plays an important role in the protection of intestinal health, and several factors such as antibiotics, infections, and stress will cause dysbiosis and different pathologies in the gut. LF is an iron-containing glycoprotein that is able to compete with pathogens for iron ions and generate electrostatic attraction with bacterial cell membrane components, contributing to the modulation of gut microbiota. The efficacy of lactoferrin as a selective modulator of the microbiome has been established in several animal models and human studies. (source)
Lactobacillus, a predominant genus in the small intestine, improves nutrient absorption, alleviates inflammatory responses by reducing the expression level of TNF-α in a rat colitis model, prevents the infection or colonisation of pathogens by competing for epithelial binding sites and nutrients, and also produces antimicrobial factors, such as bacteriocins and lactic acid. (source)
Early life LF intervention in suckling piglets, increases the abundance of Bacteroidetes and decreases the levels of Proteobacteria and Fusobacteria. The bacteroidete phylum contains many SCFA-producing bacteria, such as acetic-producing or butyrate-producing Prevotella and Bacteroides, which preserve intestinal health. The Fusobacteria phylum, which includes the Fusobacterium genus, is closely related to human colon cancer, ulcerative colitis, and other diseases such as lameness and facial skin necrosis in piglets. (source)
The Proteobacteria phylum includes various pathogens, such as Escherichia-Shigella, Salmonella, and Helicobacter. Escherichia-Shigella impairs the intestinal electrolyte balance and decreases the absorption of fluids, conditions that lead to intestinal dysfunction. It has been reported by several authors that the levels of these iron-dependent, Gram-negative bacteria are reduced in the intestines of piglets and/or gilts after supplementing diet with lactoferrin.
Antibacterial Properties
Although the aetiology of inflammatory bowel diseases (IBD), such as Crohn’s and ulcerative colitis, remains unknown, there is evidence that an abnormal immune response against the microorganisms of intestinal microbiota can be one of the causes, the adaptive and innate immune responses being involved. Moreover, some pathogenic bacteria, fungi, viruses, and parasites have been suggested to be involved in the development and exacerbation of IBD.
The bacteria most frequently associated with IBD are Mycobacterium avium subspecies paratuberculosis, Clostridium difficile, Listeria monocytogenes, and Escherichia coli, among others. In particular, a pathovar of E. coli called AIEC (adherent-invasive E. coli) has been strongly associated with many Crohn’s diseases. This pathogen is able to adhere to intestinal cells, to invade them and to replicate in epithelial cells and macrophages, eventually producing intestinal diseases in humans.
The most common viruses causing intestinal disorders are rotavirus and norovirus. The antiviral activity of bLF against the simian rotavirus, SA-11, was evaluated in the human colon adenocarcinoma cell line HT-29 by Superti et al.. They evaluated the activity of LF after saturation with different metals. The results showed that the antiviral activity of LF, fully saturated with manganese or zinc, diminished to some extent, compared to that observed for apo-or iron-saturated LF. The antiviral activity of differently metal-saturated LF towards rotavirus was exerted during and after the virus attachment step.
They also evaluated the activity of LF after removal of sialic acid, which, surprisingly, improved the anti-rotavirus activity of LF. Furthermore, when studying the effect of LF hydrolysis with trypsin, on its antiviral activity, they found that a large fragment (86–258) and a small peptide (324–329) were able to inhibit rotavirus, although to a lower extent than undigested LF.
Alex is a certified Functional Medicine Practitioner (IFMCP) and has a MSc in Personalised Nutrition. He is also a breathwork facilitator with a background in personal training and massage therapy. He also runs The Resiliency Program - a 24 week program aimed at building physical, mental, emotional, and spiritual resilience.